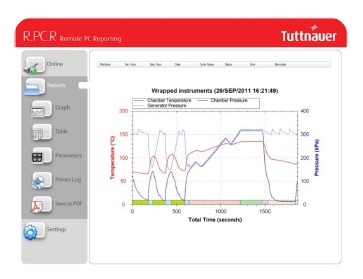
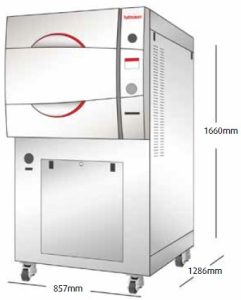
Overview
A free-standing, cabinet enclosed unit is simple and as easy to use as any tabletop autoclave. The 5075 HSG is a hospital grade Class B autoclave, suitable for large medical & dental clinics, and operating rooms. Designed to be a fast cycle sterilizer that is mobile, operates 24 hours a day, and can optionally operate independent of building utilities.
Packed with all the features and reliability of a large capacity autoclave at a fraction of the size and cost, the 5075 HSG now comes with with Virus Protective Shield, a virus pre-sterilization cycle.
- 160 Liter chamber
- Easy to use. 36 Multi-colored display and easy 3-button pad to easily browse pre-programmed sterilization cycles and user directories with a built-in alpha-numeric printer
- Fast & efficient. Coiled jacket increases overall cycle speed, performance and ensures uniform heat distribution throughout the chamber
- Safe. Double locking safety device prevents door from opening at high pressure and high temperature
- High quality 316 L stainless steel chamber and door with electro-polish finish
- Rapid air removal. High volume water-ring vacuum pump for fast and efficient air removal.
- Easy to move & install, Mounted on wheels for easy mobility. Connects to utilities with flexible piping for an easy installation